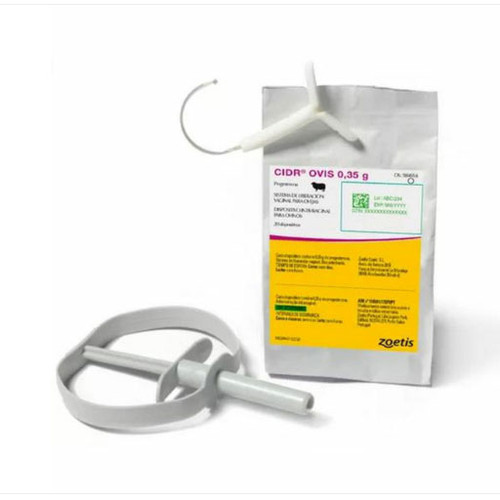
CIDR 1.38g Vaginal Delivery System for Cattle.
Presentation
CIDR is presented as a "T" shaped device consisting of a progesterone impregnated silicone elastomer moulded over an inert nylon spine. Each device contains 1.38 g progesterone.
Uses
For the control of the oestrous cycle in cycling cows and heifers, including:
- Synchronisation of oestrus in groups of animals.
- Synchronisation of donor and recipient animals for embryo transfer.
To be used in combination with prostaglandin F2a or analogue.
Use as recommended normally results in oestrus 48-96 hours after device removal with the majority of animals showing oestrus within 48-72 hours.
For induction and synchronisation of oestrus in Fixed Time Artificial Insemination (FTAI) protocols:
- In cycling cows and heifers. To be used in combination with prostaglandin F2a (PGF2a) or analogue.
- In cycling and non-cycling cows and heifers. To be used in combination with Gonadotropin releasing hormone (GnRH) or analogue and PGF2a or analogue.
- In non-cycling cattle. To be used in combination with PGF2a or analogue and equine chorionic gonadotrophin (eCG).
Dosage and administration
1.38g of progesterone (1 device) per animal for 7 - 9 days (depending on indication).
For synchronisation of oestrus and synchronisation of donor and recipient animals for embryo transfer:
One device should be inserted into the vagina of each cow or heifer to be treated. The vaginal insert should be left in position for 7 days with an injection of a luteolytic dose of prostaglandin F2a or analogue administered 24 hours prior to removal. In animals that respond to treatment the onset of oestrus generally occurs within 1-3 days after removal of the insert. Cows should be inseminated within 12 hours of first observed oestrus.
For the induction and synchronisation of oestrus for Fixed Time Artificial Insemination (FTAI):
The following FTAI protocols have been commonly reported in the scientific literature and should be used:
In cycling cows and heifers
- Insert one CIDR 1.38g into vagina for 7 days.
- Inject a luteolytic dose of PGF2a or analogue 24 hours prior to device removal.
- FTAI 56 hours after removal of the device.
In cycling and non-cycling cows and heifers
- Insert one CIDR 1.38g into vagina for 7- 8 days.
- Inject a dose of GnRH or analogue at CIDR 1.38g insertion.
- Inject a luteolytic dose of PGF2a or analogue 24 hours prior to device removal.
- FTAI 56 hours after removal of the device, or
- Inject GnRH or analogue 36 hours after CIDR 1.38g removal and FTAI 16 to 20 hours later.
In non-cycling cows
The following FTAI protocol should be used:
- Insert one CIDR 1.38g into vagina for 9 days.
- Inject a luteolytic dose of PGF2a or analogue 24 hours prior to device removal.
- Inject eCG at CIDR 1.38g removal.
- FTAI 56 hours after removal of the device, or inseminate within 12 hours following first observed oestrus behaviour.
Administration
A device applicator should be used for administration, following the procedure described below:
1.Ensure that the applicator is clean and dipped in a non-irritant antiseptic solution before use.
2.Wearing sterile disposable plastic gloves, fold the arms of the device and load into the applicator. The arms of the device should protrude slightly from the end of the applicator. Care should be taken to avoid unnecessary or prolonged handling of the product to minimise transfer of the active substance to the operator's gloves.
3.Apply a small quantity of obstetrical lubricant to the end of the loaded applicator.
4.Lift the tail and clean the vulva and perineum.
5.Gently insert the applicator into the vagina, first in a vertical direction and then horizontally until some resistance is encountered.
6.Make sure the removal string is free, press the handle of the applicator and allow the barrel to move back towards the handle. This releases the arms of the device, which will then retain the device in the anterior vagina.
7.With the device correctly positioned, withdraw the applicator, leaving the removal string hanging from the vulva.
8.The applicator should be cleaned and disinfected before being used on another animal.
Removal
The device may be removed by gently pulling on the string. On occasions the string may not be visible from the outside of the animal, in such cases it may be located in the posterior vagina using a gloved finger. Withdrawal of the device should not require force. If any resistance is encountered a gloved hand should be used to ease removal.
If there is any difficulty in removal from the animal beyond that itemised above veterinary advice must be sought.
The device is intended for single use only.
Contra-indications, warnings, etc
Do not use in cows or heifers with abnormal or immature genital tracts, or with genital infections. Do not use in pregnant cattle. Do not use within the first 35 days after calving.
Animals in poor condition, whether from illness, inadequate nutrition or other factors may respond poorly to treatment.
Vaginal discharge associated with local irritation has been observed at removal of the insert. This discharge generally clears between the time of removal and insemination and does not affect fertility at inseminations following treatment. Can be used during lactation. Laboratory studies in rat and rabbit, after intramuscular or subcutaneous administrations, and at repeated high doses of progesterone, have produced evidence of foetotoxic effects.
Withdrawal periods:
Meat & offal – zero days.
Milk – zero days.
During treatment, milk can be used for human consumption.
Operator warnings:
Personal protective equipment consisting of gloves should be worn when handling the veterinary medicinal product during insertion and removal. Insert the device using the applicator.
Wash hands and exposed skin with soap and water after use. Do not eat, drink or smoke while handling the product.
Pharmaceutical precautions
Do not store above 30°C.
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal products should be disposed of in accordance with local requirements.
Keep out of the sight and reach of children.
For animal treatment only.
Legal category
POM-V
Packaging Quantities
CIDR is supplied in heat-sealed low density polyethylene sachets containing 10 devices.
Shipping charges mainland GB zone1
| Value | Weight | Delivery Charge |
| Over £49.00 | Any Weight | FREE |
| Under £49.00 | No weight limit | £4.50 |
| Under £49.00 | Under 250 grams | £3.49 |
Most orders are despatched the same day and standard delivery is 3 to 5 working days from despatch. FAST Expedited options are available at extra cost - these usually arrive in 24-48hrs from dispatch but delays are possible during busy periods.
Postcodes outside of Zone 1 (including some areas within mainland UK) may incur extra carriage charges due to surcharges imposed by the couriers. These will be calculated on the website at the checkout.
NB: Orders placed with faster delivery must be placed before 13:00 to ensure same day dispatch (excluding weekends and bank holidays). Please also check on the product page that the items ordered show 'In Stock' otherwise your order will be delayed until the stock becomes available, which in most cases will only be 24hrs.
Estimated "Usually shipped in" information is shown against most products
Overseas orders
We no longer send goods outside of the UK.
Prescription Items
Prescription items will be sent on receipt of a valid signed and dated prescription.
Pick up
We offer a pickup service for orders placed over the phone.
Please ring us on 01833 641112 for more information.
Refrigerated Items
Refrigerated items will be sent by carrier or express delivery at a cost of £7.50, with coolpacks, unless you pay for a premium for a refrigerated delivery. We will contact you prior to despatch for your instructions.
We want you to be completely satisfied with any purchase. If not any item* can be returned to us within 14 days of receipt for exchange or refund Please give us a ring for a returns number on 01833 641112 or alternatively email sales@hyperdrug.co.uk to obtain a returns number.
*Due to legal requirements medicines may not be returned except to correct an error in despatch or in response to an "official recall". If an item is believed to be faulty it should be returned for inspection and it may be necessary to forward it to the manufacturers for testing before replacements or refunds can be authorised. This does not infringe legal rights. Please contact our customer services for a "Returns Number" which must appear on the outside of the package or it will not be accepted. We advise customers to use an insured method of shipping and to retain proof of despatch. We may refuse returns on products specially obtained or manufactured to order. Items must be returned unsoiled and unused and sent adequately packed and carriage paid.